Introduction
As part of my mission at Sanofi, I designed and developed an algorithmic simulation platform to optimize the management of clinical investigational product supplies. This solution addresses the critical challenges of clinical trial supply chains, where the complexity of global distribution and patient visit management represent major operational issues with direct implications on study continuity and success.
The solution provides supply chain teams with predictive analytics and complex scenario simulation capabilities, enabling proactive identification of supply risks and adaptation of distribution strategies in line with the evolving needs of clinical studies.
Strategic Context and Business Challenges
Managing the clinical trial supply chain is facing increasing operational complexity. The main challenge lies in preventing patient missed visits, which can compromise the integrity of study data and delay the development of new treatments. These crisis situations require immediate reaction capabilities and full visibility on the availability of investigational products across the entire network of clinical sites.
Beyond crisis management, optimizing distribution significantly reduces the waste of expensive investigational products while ensuring their continuous availability for every patient. This complex equation is amplified by the diversity of IRT (Interactive Response Technology) systems in use, each provider imposing its own data formats, allocation rules, and operational processes.
In this context, my scope focused on two major IRT providers used by Sanofi: ENDPOINT and Suvoda. These systems, while essential for trial management, operated in silos, limiting cross-study analytical capabilities and making it difficult to anticipate supply risks. The absence of a consolidated view impaired decision-making efficiency and increased reaction times in critical situations.
Sanofi already uses the N-SIDE solution for strategic planning of the clinical supply chain. However, this platform operates at a high level of aggregation (depot, country, mid- to long-term horizon) and does not directly work at the operational granularity of IRT exports (patient visit, site, kit type, lot). It is very effective for global sizing and optimization questions, but less suited to rapid diagnostics and fine-grained simulation close to the actual IRT data.
Project Scope and Strategic Approach
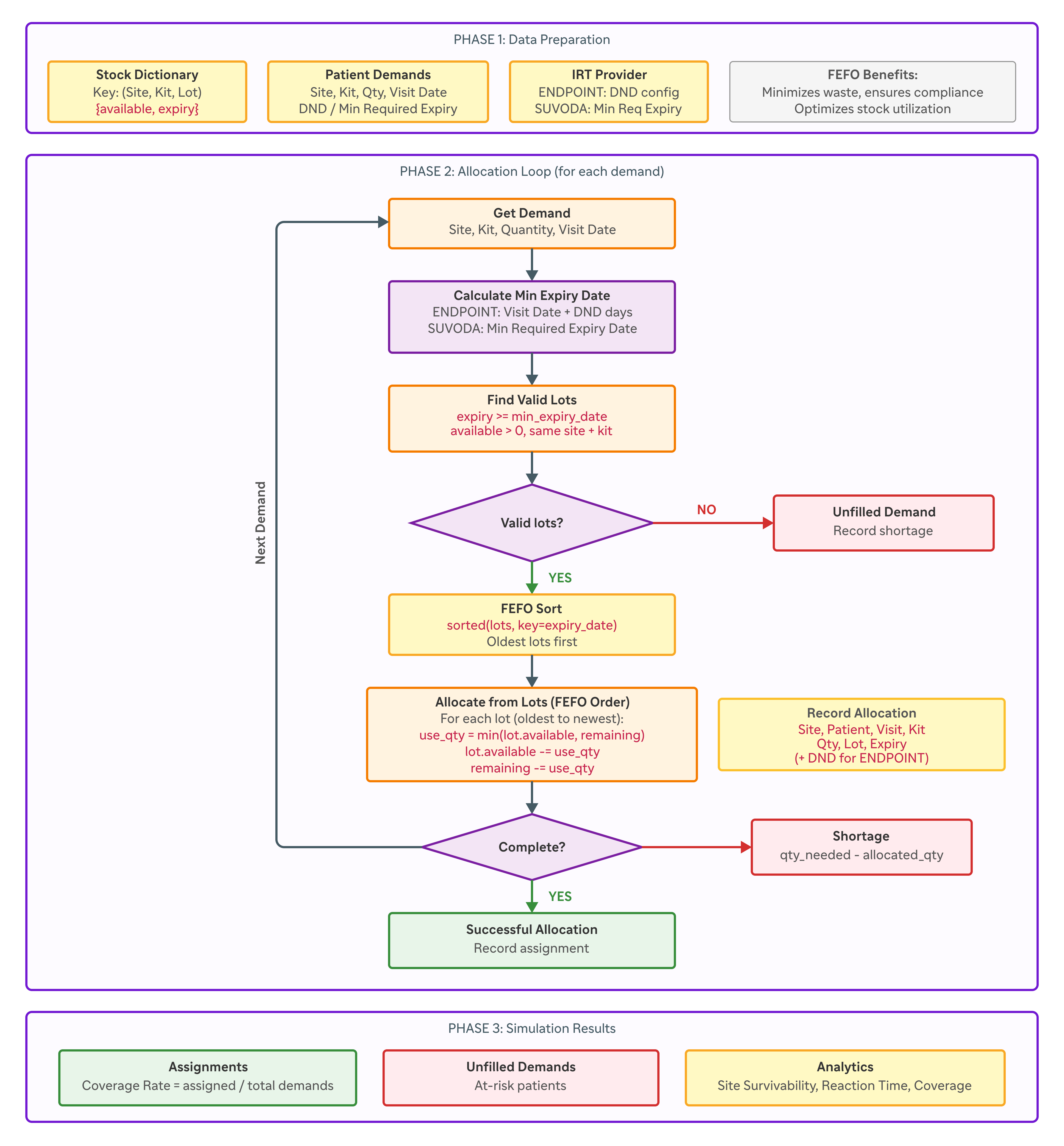
The project aimed to create a unified platform able to simulate the allocation of clinical investigational products according to the FEFO (First Expired, First Out) algorithm, independently of the source IRT system. The chosen approach favored agility and adaptability, enabling rapid implementation while anticipating future evolutions.
The POC is positioned as a complement to N-SIDE: where N-SIDE produces global optimizations that require a heavier modeling and computation cycle, the simulation platform provides a fast, low-cost view that can be re-run on demand. Clinical Supply Chain Managers can test different allocation and shipment strategies themselves, without waiting for a new N-SIDE study run.
Operating within the Business division of the Clinical Supply Chain department, I designed an architecture that turns limited access to central systems into a competitive advantage. The solution relies on a data import–based approach, enabling immediate adoption without depending on complex IT infrastructures, while maintaining the rigor required for future enterprise integration.
This strategy made it possible to demonstrate the operational value of the solution quickly, creating momentum for the digital transformation of the clinical supply chain without requiring upfront infrastructure investments.
Algorithmic Architecture and Technical Innovation
FEFO Algorithm Implementation
The FEFO algorithm developed is the core of the platform’s predictive capabilities. Its sophistication lies in the simultaneous handling of multiple constraints: product expiry dates, patient visit windows, distribution rules specific to each IRT provider, and safety parameters defined by study protocols.
By working directly on IRT exports, the algorithm reconstructs real stock behavior at a level of granularity that N-SIDE does not cover: chronological sequence of visits, impact of DND rules, and effective use of lots by site and kit type. This modeling precision makes it possible to analyze concrete incidents and very quickly test realistic mitigation strategies (changing shipment prioritization, adjusting rules, targeted redeployment).
The algorithmic architecture implements a two-phase approach. The first phase performs a full simulation of future allocation based on planned visits and available inventory. The second phase applies optimization strategies to explore different resupply scenarios and identify optimal configurations to minimize risk.
This approach ensures full compliance with GxP regulatory requirements and WHO recommendations, while offering the flexibility needed to adapt to the specificities of each clinical study.
Multi-Provider Architecture
The architecture developed bridges the differences between ENDPOINT and Suvoda through a sophisticated abstraction pattern. ENDPOINT uses a model based on complex visit windows with configurable DND (Do Not Dispense) rules, while Suvoda relies on pre-calculated minimum expiry dates requiring intelligent aggregation of units.
The automatic detection system analyzes the structure of incoming files and applies the appropriate transformations into a unified data model. This approach removes complexity for the end user while preserving the richness of provider-specific information.
Interface and User Experience
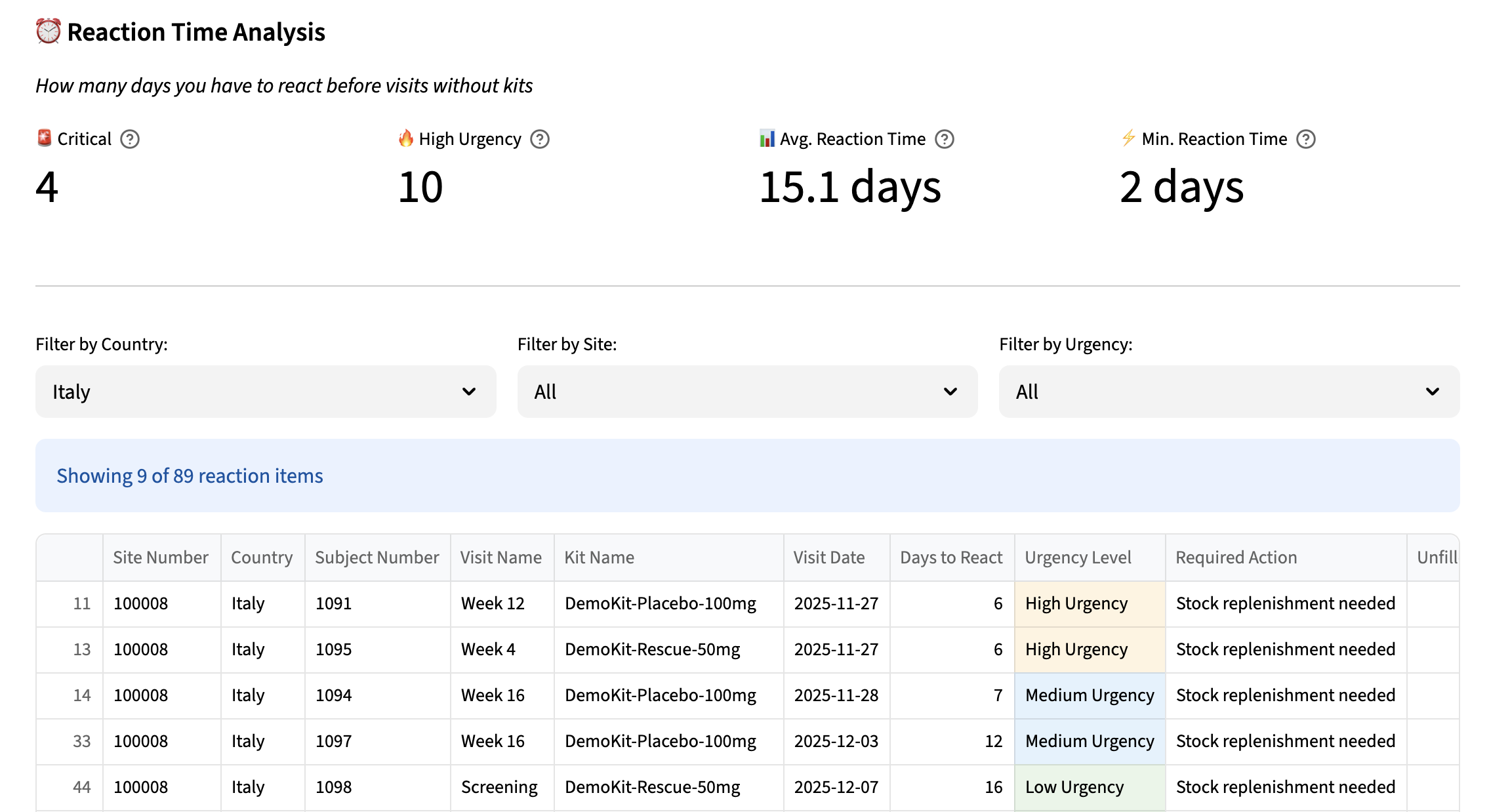
The platform offers an analytical interface designed to turn data complexity into actionable insights. The dashboard synthesizes the state of the supply chain through intuitive visualizations, enabling immediate identification of risk areas and optimization opportunities.
Interactive simulation capabilities allow users to quickly explore different scenarios, assessing the impact of changes in resupply strategies or allocation parameters. This what-if approach transforms supply chain planning from a reactive process into a proactive and strategic one.
The system automatically generates detailed reports compatible with existing processes, facilitating communication with stakeholders and accelerating decision-making in crisis situations.
Technology Choices and Technical Architecture
Constraints of the Business Division environment led to a technology stack focused on efficiency and portability. Python naturally emerged as the backend development language, offering a rich ecosystem of scientific libraries while remaining accessible to Sanofi’s data science teams. Pandas and NumPy form the core of data processing, enabling efficient manipulation of large volumes with optimal performance.
Streamlit was selected for the user interface, providing a framework for rapid prototyping of data-centric applications with minimal code. This approach significantly accelerated the development cycle while delivering a professional, responsive interface.
Deployment is handled through Posit Connect, the analytical application publishing platform already established in Sanofi’s ecosystem. This choice guarantees compliance with enterprise security standards while simplifying access management and maintenance. Native integration with the existing infrastructure facilitated adoption by teams and reduced deployment friction.
Despite environmental constraints, this technical architecture demonstrates that it is possible to build enterprise-grade solutions by optimizing the use of available tools and prioritizing architectural excellence over technological complexity.
Impact and Outlook
Demonstrated Results
The proof of concept validated the relevance of the approach by demonstrating substantial operational gains. Analyses that previously required several days of manual work are now executed in a few minutes, radically transforming teams’ ability to react in critical situations.
The platform offers a new capability for real-time crisis management, enabling instant evaluation of multiple allocation scenarios and dynamic adaptation of distribution strategies. This operational agility represents a significant competitive advantage in an environment where each day of delay can compromise the development of a new treatment.
Although the solution has not yet been deployed on live crisis cases, simulations carried out on historical data have demonstrated its ability to proactively identify risks and propose relevant optimization strategies.
Evolution Towards an Enterprise Solution
The success of the POC has sparked strong interest from Sanofi’s specialized IRT teams, who are planning the evolution of the solution into an integrated production platform. This transition includes establishing direct connections with IRT system APIs and extending coverage to all providers used globally.
The modular architecture developed supports this evolution, allowing new features to be added progressively and integration with central systems without major redesign. This approach validates the initial vision of an evolutive solution capable of adapting to the organization’s growing needs.
The integration with N-SIDE is natural: N-SIDE retains its role as a strategic optimization and global sizing engine, while the IRT simulation platform adds a much more responsive visualization and scenario-testing layer, directly usable by CSCPM teams. This combination provides the best of both worlds: an optimized long-term view and a near–real-time operational capability, without the overhead of additional heavy computation.
Conclusion
This project illustrates how architectural excellence and algorithmic innovation can create transformative operational value, even in a constrained environment. The ability to turn limitations into innovation opportunities made it possible to develop a solution that not only meets immediate needs but also lays the foundations for a broader transformation of the clinical supply chain.
The planned evolution towards an enterprise solution demonstrates the robustness of the architecture and its strategic relevance. This experience reinforces the belief that innovation emerges from aligning technical excellence with a deep understanding of business challenges, creating solutions that transcend constraints to generate lasting value.